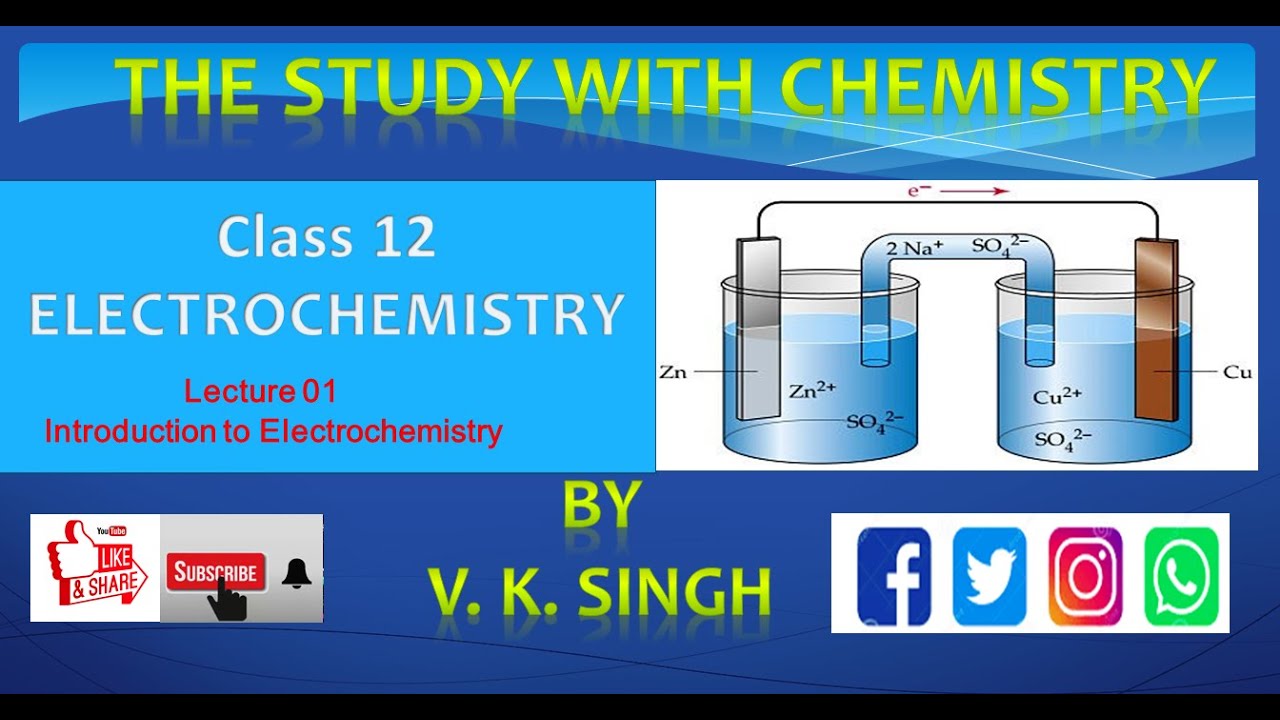
Class 12, Lecture01, Introduction to Electrochemistry YouTube
These metallic electrodes are immersed in an electrolytic solution for power generation. By thorough reading of chapter 3 Chemistry class 12 notes, students will know that the ionic conductor is a vital part of cells. Download CBSE Class 12 Chemistry Notes 2023-24 PDF. Also, check CBSE Class 12 Chemistry revision notes for other chapters:

ELECTROCHEMISTRY PART3 CLASS12 YouTube
This portion of power point presentation on electrochemistry includes, electrolytic conduction, conductivity, cell constant, molar conductivity, variation with concentration & temperature, Debye Huckel Onsager equation, Limiting molar conductivity, Kohlrausch law of independent migration of ions, its application & numerical problems.
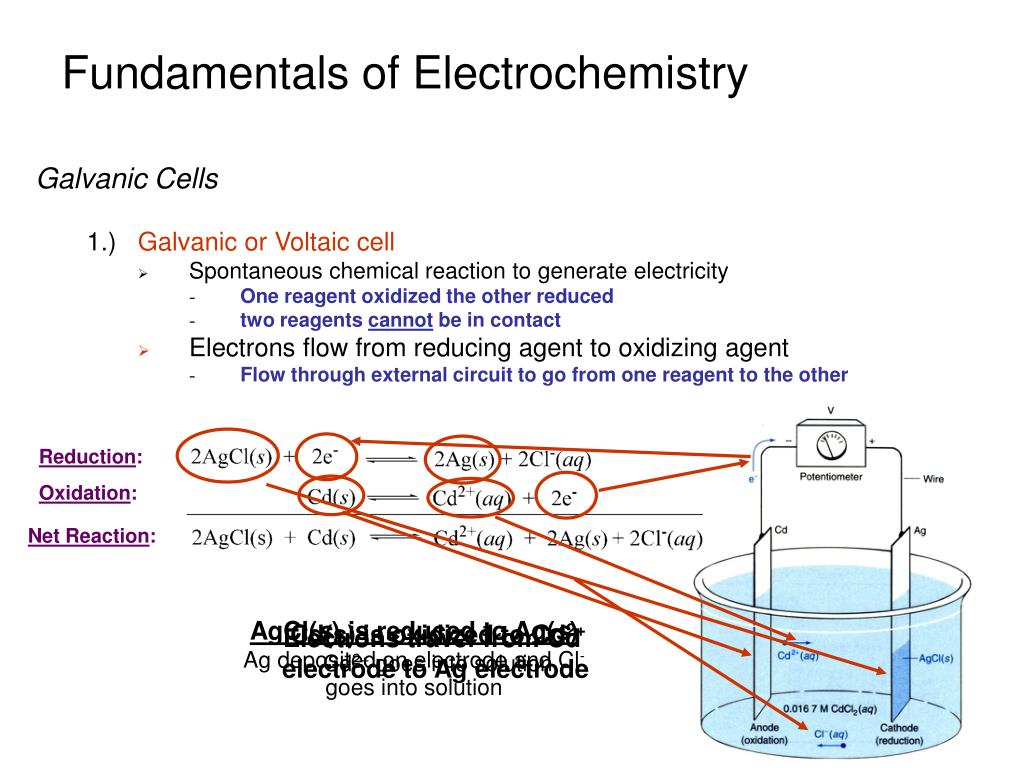
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free
Electrochemistry class 12 Apr 11, 2018 • 65 likes • 58,226 views Cleophas Rwema Education Electrochemistry Electrochemistry class 12 1 of 68 Download Now Save slide Save slide Recommended d and f block elements Tinto Johns Vazhupadickal 66.2K views • 75 slides Classification of elements and periodicity in properties class 11th chapter 3. ritik
CLASSNOTES Electrochemistry Notes For Class 12 Free Download
Download revision notes for Electrochemistry class 12 Notes and score high in exams. These are the Electrochemistry class 12 Notes prepared by team of expert teachers. The revision notes help you revise the whole chapter 3 in minutes. Revision notes in exam days is one of the best tips recommended by teachers during exam days.

electrochemical cell electrochemistry class 12 chemistry subject notes
Class 12 Chemistry (India) 9 units · 66 skills. Unit 1 The Solid State. Unit 2 Electrochemistry. Unit 3 Chemical kinetics. Unit 4 The p-block elements. Unit 5 The d-block elements. Unit 6 Coordination compounds. Unit 7 Haloalkanes & haloarenes. Unit 8 Alcohols, phenols, and ethers.
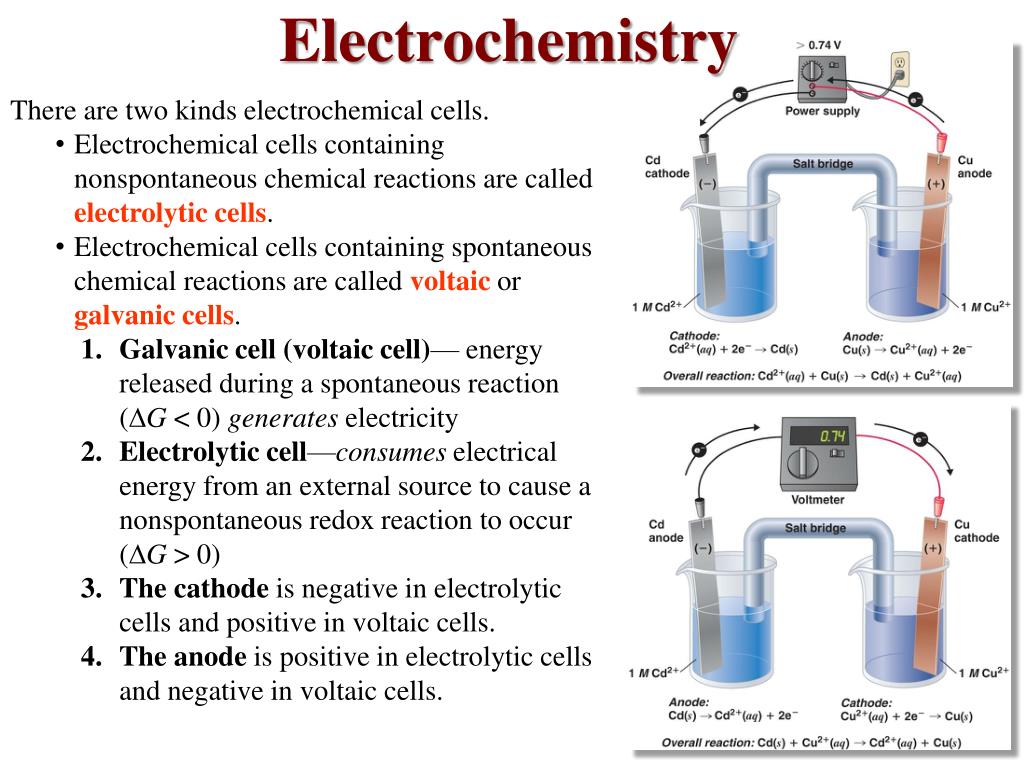
Ppt Basic Electrochemistry Powerpoint Presentation, Free Download E45
These are two fundamental types of electrochemical cells. Galvanic cell - In a galvanic cell, spontaneous redox reaction, the chemical energy is converted into electrical energy. It is also called a voltaic cell or Daniel cell. Electrolytic cell - In an electrolytic cell, the nonspontaneous redox reaction is carried out by electrical energy.

Electrochemistry class 12 notes [PPT Powerpoint]
anode positive. A student places a copper electrode in a 1 M solution of CuSO4 and in another beaker places a silver electrode in a 1 M solution of AgNO3. A salt bridge composed of Na2SO4 connects the two beakers. The voltage measured across the electrodes is found to be + 0.42 volt. (a) Draw a diagram of this cell.

2. Electrochemistry Class 12 Board Standard Hydrogen Electrode
12th Chemistry PowerPoint Presentation Materials (Complete PPT Materials) Mr. B. Uthrakumar. Unit 1 PPT ( Metallurgy) - English Medium - Preview & Download (MAT.NO. 219313) Unit 2 PPT (p Block Elements I) - English Medium - Preview & Download (MAT.NO. 219394) Unit 3 PPT (p Block Elements II) - English Medium - Preview & Download.

Chapter 3 Electrochemistry Class 12 Handwritten Notes PDF download
In Class XI, Unit 8, we had studied the construction and functioning of Daniell cell (Fig. 3.1). This cell converts the chemical energy liberated during the redox reaction Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) (3.1) to electrical energy and has an electrical potential equal to 1.1 V when concentration of Zn2+ and Cu2+ ions is unity (1 mol dm-3)*.

PPT AP Chemistry Unit 12 Electrochemistry PowerPoint Presentation
Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry Production of metals like Na, Mg. Ca and Al. Electroplating.

Electrochemistry Class 12 Important Questions with Answers
24 May 2023 by Chetan Sharma Download links for Class 12 chemistry ppt for all chapters. In this article, we are going to provide you with the ppt of all chapters from class 12 chemistry. These ppts are very helpful for class representations. These ppts can also be used as class 12 chemistry quick revision notes for preparation for exams.

Electrochemistry class 12
Class XII Electrochemistry - Download as a PDF or view online for free. Submit Search. Upload. Class XII Electrochemistry . Report. Share. Arunesh Gupta Govt. service at KVS, New Delhi.. Chapter 8 redox reactions ppt for class 11 CBSE by .

Electrochemistry class 12
PPT: Electrochemistry - Chemistry Class 12 - NEET Download, print and study this document offline Download as PDF Up next NEET Previous Year Questions (2014-23): Electrochemistry NCERT Solutions: Electrochemistry Flashcards: Electrochemistry Top Courses for NEET Biology Class 11 NEET Mock Test Series Topic-wise MCQ Tests for NEET Physics Class 11

Electrochemistry Class 12 Mind Map
NCERT Solutions for Class 12 Chemistry Chapter 3 - Free PDF Download. NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry play a pivotal role in the CBSE Class 12 Chemistry board examination.NCERT Solutions for Class 12 Chemistry are comprehensive materials that have answers to the exercise present in the NCERT Textbook. These solutions are developed by subject experts at BYJU.

Chapter 3 Electrochemistry Class 12 Handwritten Notes PDF download
Arunesh Gupta 2.9K views • 31 slides ) Similar to CBSE Class 12 Chemistry Chapter 3 (Electrochemistry) | Homi Institute 20 More from Homi Institute (9) CBSE Class 12 Chemistry Chapter 3 (Electrochemistry) | Homi Institute - Download as a PDF or view online for free
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID4385248
Electrochemistry Electrolysis ' Electrolysis: is the process in which electrical energy is used to drive a nonspontaneous chemical reaction. An electrolytic cell is an apparatus for carrying out electrolysis. ' Processes in an electrolytic cell are the reverse of those in a galvanic cell. Slide 2